Research
- Home
- Research
About the STIM Lab
High-Throughput Phenotyping
Our research focuses on high throughput phenotyping: building three-dimensional macroscopic models of tissue at sub-cellular resolution.
The STIM laboratory develops and uses a wide range of tools, primarily in the areas of optical imaging and high-performance computing. Major areas of research include:
Fluorescence Microscopy. We routinely build microscopes for wide-field and three-dimensional imaging applications, including confocal, multi- photon, and light-sheet microscopy.
Image Segmentation. We develop methods that turn three-dimensional images into explicit models for analysis, including segmentation of complex structures such as neurons and microvascular networks.
Visualization. We employ a wide range of custom tools to visualize unique structures, such as networks and large data volumes, and have expertise in computer graphics programming and virtual reality.
GPU Programming. We frequently leverage CUDA programming for both image processing and visualization, and the PI (Dr. Mayerich) teaches a graduate course on heterogeneous computing.
Optical Modeling. We use physically-based modeling to understand the interactions between materials and electromagnetic fields. This includes developing methods to iteratively reconstruct samples based on EMF scattering and absorbance.
Publications
Data
Most of these data sets are shared using Resilio Sync keys. This allows us to overcome data size constraints using peer-to-peer transfers via BitTorrent. Download Resilio sync and use the provided key to access the associated data set.
To help with data transfers, it would be great if you continued sharing the data after acquiring it!
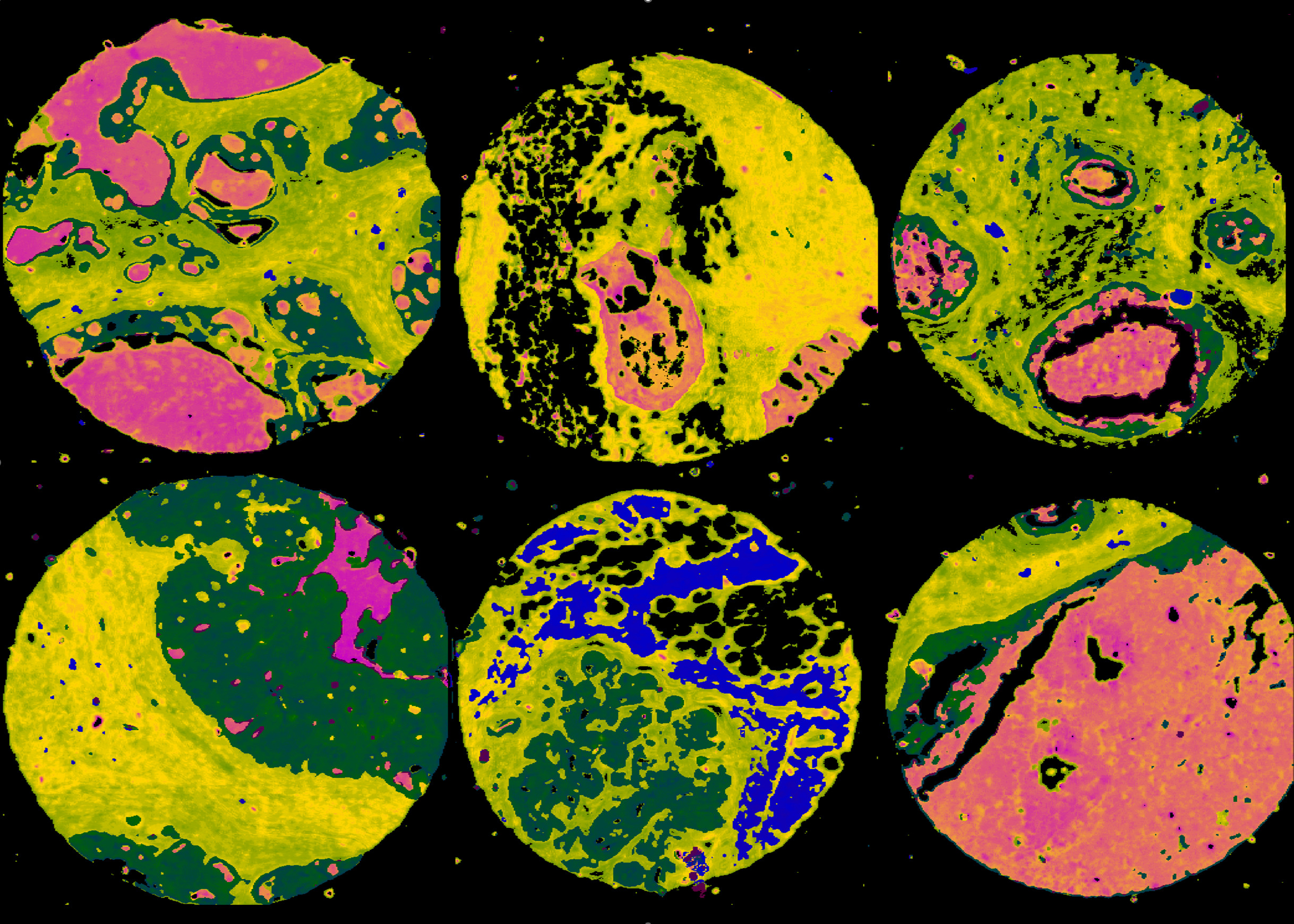
Fourier Transform Infrared Spectroscopy
The following tissue microarrays were collected using an Agilent Cary 620 FTIR microscope.
Biomax Breast Cancer Arrays (High-Definition)
Collected by Dr. Michael Walsh in Rohith Bhargava’s lab at UIUC. The images were collected using an Agilent Cary 620 FTIR imaging system. The system was modified using a 74X (0.65 NA). Each repository consists of:
● Raw binary data with an associated ENVI header file
- ● Widefield images of adjacent stained sections
- ● Annotations of selected cell types
We also provide a link to the most closely related Biomax tissue microarray that is available for purchase.
BR961a Breast Cancer Array
Resilio Key: B7LKHU7NUH2BVHLY67246T7ERBIU2SU4K
(Biomax Array)
BR1003a Breast Cancer Array
Resilio Key: BNXNJI3C2UEG23KY3KAO6LNBXHBDCQVG2
(Biomax Array)
Biomax Breast Cancer Arrays (Standard-Definition)
Collected by Dr. Michael Walsh in Rohith Bhargava’s lab at UIUC. The
images were collected using an Agilent Cary 620 FTIR imaging system using a 15X (0.5 NA) objective. Each repository consists of:
● Raw binary data with an associated ENVI header file
- ● Widefield images of adjacent stained sections
- ● Annotations of selected cell types
We also provide a link to the most closely related Biomax tissue microarray that is available for purchase.
BR961a + BR1001 Breast Cancer Array
Resilio Key: BYVNK2L7GVGCCOTNPTLALZVMH7J3JIESQ
(Biomax BR1003a, BR2085d)
BR1003a + BR2085b Breast Cancer Array
Resilio Key: BNULKO33XIUDOZZMVPQQ7NZWDTWGBL2NM
(Biomax BR961a, BR1001)
Instrumentation
Imaging Systems
Galvo/Resonant Confocal Microscope. Upright galvo/resonant confocal imaging system equipped with 4 high-sensitivity photomultiplier tubes for multiplex imaging of large samples.
Galvo/Galvo Confocal Microscope. Inverted single-channel galvo/galvo confocal scanning system with an adjustable dwell time for low-signal 3D imaging.
Widefield Fluorescence Microscope. Inverted Nikon Ti microscope with a tunable excitation system and filter bank for 10+ plex widefield fluorescence microscopy.
Lightsheet Microscope. ASI dual-plane selective plane illumination microscope (diSPIM).
Two-Photon Microscope. Bergamo II multi-photon microscope with a tunable Ti:Sapphire femtosecond laser.
FTIR Microscope. Agilent Cary 620 infrared microscope with a 630 spectrometer and a 128×128 MCT focal plane array for imaging.
Prototype Systems
OCTLS. Dual-mode imaging system capable of simultaneous optical coherence tomography (OCT) and lightsheet microscopy. The lightsheet module can produce both single-photon and dual-photon excitation.
MUVitome. Custom 3D milling with ultraviolet excitation (MUVE) imaging system for imaging large 3D samples using serial ablation.
Support Systems
Optics and Optomechanics. We are a fully-equipped optics lab with a wide range of components including lenses, filters, coherent and incoherent light sources, and optomechanical mounts.
Histology Laboratory. Our histology lab has equipment necessary for tissue processing, staining, staining, and immunofluorescence. This includes incubators, refrigerator, freezer, vaccine (-70C) freezer, microtome, and a wide array of primary/secondary antibodies and fluorophores.
Stochastic Electrotransport. We have a LifeCanvas SmartLabel system for clearing and immunofluorescent labeling of large samples, such as whole rodent organs.
3D Printer. Formlabs Form3 SLA printer with a variety of polymers.
Software
Our lab maintains a public GitHub repository (STIM-Lab) for source code. Published tools and algorithms will be available there. We use vcpkg for package management and leverage public libraries as much as possible. All software should work with CMake and is tested on Windows and Linux (Ubuntu LTS). A tutorial for our build pipeline is available here.